
There’s dew on the grass now, above the swamp. It was a given after a such a beautiful sunny day. No clouds, sun sets, temperature plummets. That’s the way it goes in Upper Plenty in spring. But the two men knee deep in the swamp have come prepared. Woolen beanies, heavy pants and stout jackets were the first items that went into the car. Across the swamp, where the frigid night air flows over the warm water, mists are drifting like currents among the Water Ribbon and Mud Dock. And therein the frogs are calling. The chorus rises and falls with a mysterious synchrony, as hundreds of Southern Bell Frogs float and call and squabble. Bill Sherwin – a newly minted PhD student at the University of Melbourne – is on a steep learning curve, watching his supervisor Murray Littlejohn bag frog after frog. Yet the frogs call on, near oblivious to these strange new predators and the regular alarm calls punctuating the still night air. It’s October 1976.
Four-hundred kilometers north, on the Southern Tablelands of NSW, an equivalent scene plays out. Bob Humphries is embarking on the final field season of his ambitious PhD project. In a small, spring-fed farm dam, Bob wades through dense beds of Pondweed capturing as many of the resident Southern Bell Frogs and Green and Golden Bell Frogs as his frigid limbs will allow. In spite of his rapidly fading dexterity, he needs to check for toe-clips on each and every one of these stunning green frogs to decode their population dynamics. Are they animals he’s caught and marked before? Or are they new to the population, either being recruited late in the previous summer or having immigrated from another nearby pond?
That spring in 1976 was set in a period we now recognise as the birth of Australian batrachology. Never before had such intensive studies of Australia’s frogs been attempted. Murray, Bill, Bob and notable others were trailblazers whose work represents the bedrock of what has grown to be a rich and diverse science. But that moment has other significance, for it was just prior to a calamitous event that would have sweeping impacts on Australian amphibians. That event — which extirpated the glorious green frogs of Upper Plenty and much of the Southern Tablelands — was the arrival of amphibian chytrid fungus in eastern Australia.
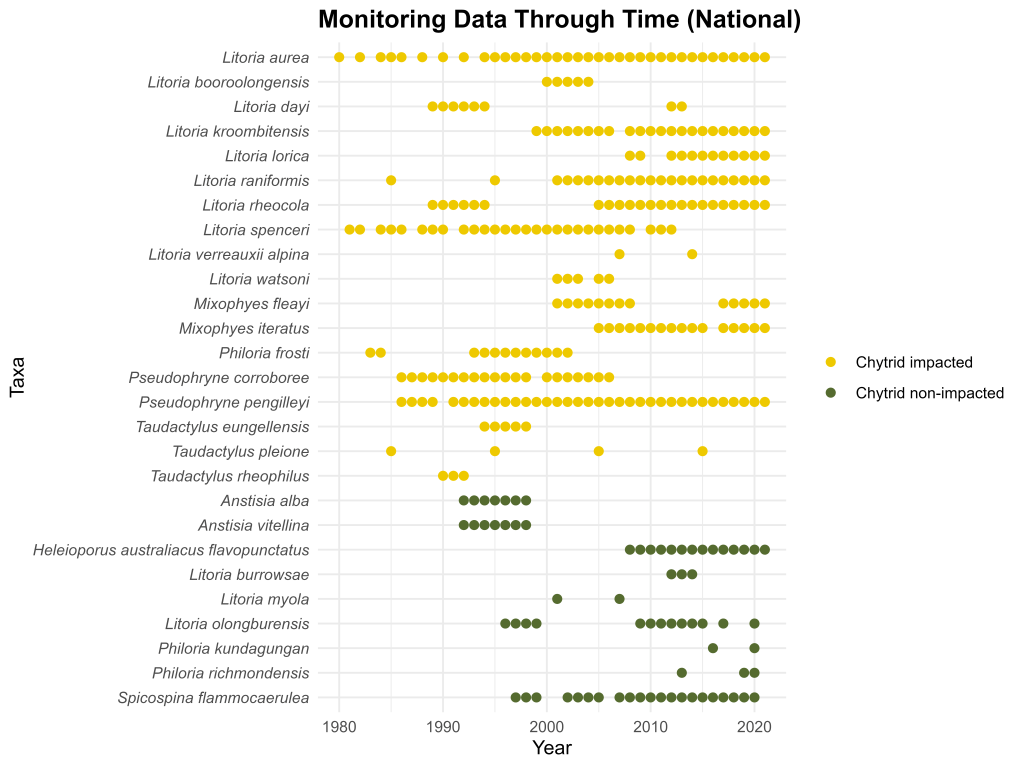
Chytrid fungus is the worst wildlife pathogen the modern world has known. In Australia, it brought millenia of evolution to a shuddering holt for 7 species, driving them into the abyss of extinction. Others went to the brink, where they remain, propped up by dedicated souls. But even those that seem to have stabilised remain profoundly affected, with diminished ranges and narrower habitat affiliations than the ones they showed before chytrid arrived. They are in an environmental stand-off with the pathogen. But why exactly?
This is a complex question to answer, but one possible reason is that populations surviving with chytrid fungus are those that can persist by offsetting higher adult mortality through other demographic mechanisms. In a recent study, myself, Ben Scheele, Michael Scroggie and Matthijs Hollanders set out to assess how such demographic mechanisms can enable persistence of frogs afflicted with endemic chytridiomycosis. We focussed on Southern Bell Frogs (SBF) and Green and Golden Bell Frogs (GGBF), knowing that the pioneering work on these frogs in the 1970s could provide powerful insights into how their demography has changed due to chytrid, and the conditions in which persistence is possible despite these profound impacts.
And so, we took ourselves off to the Museum. With the help of curators at the Melbourne Museum, the Australian Museum and the CSIRO National Wildlife Collection, we tracked down pickled SBFs and GGBFs that had been collected before chytrid arrived in Australia. We sought out specimens that were collected at sites close to those at which we’d worked on persisting populations of these frogs — Melbourne’s northern plains (a stone’s throw from Murray and Bill’s swamp at Upper Plenty) and the Southern Tablelands of NSW. We took bone samples from each specimen by removing one of their toes, just as we’d done during our contemporary sampling. And with that, we had matched bone samples from these areas pre- and post-chytrid, which we could use to age each frog and compare longevity and survival rates before and after the fungus arrived. We did this by counting lines of arrested growth (LAG) in bone cross sections, which are laid down during the winter torpor period (consider LAG the frog eqivalent of tree rings). The process is known as skeletochronology.

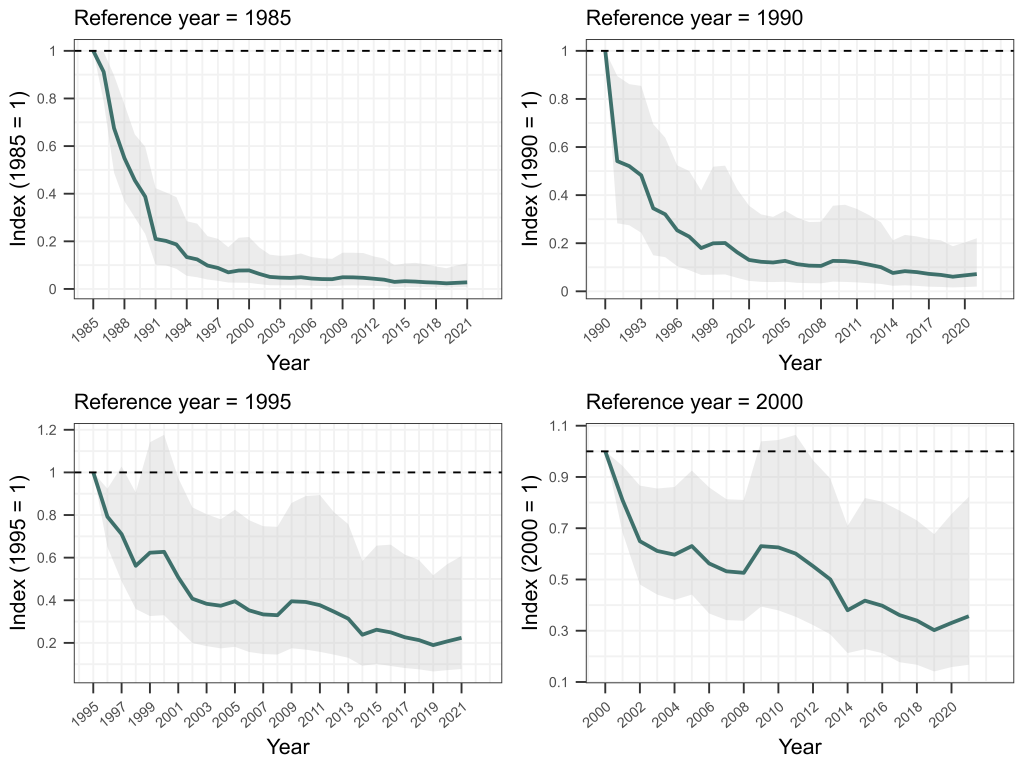
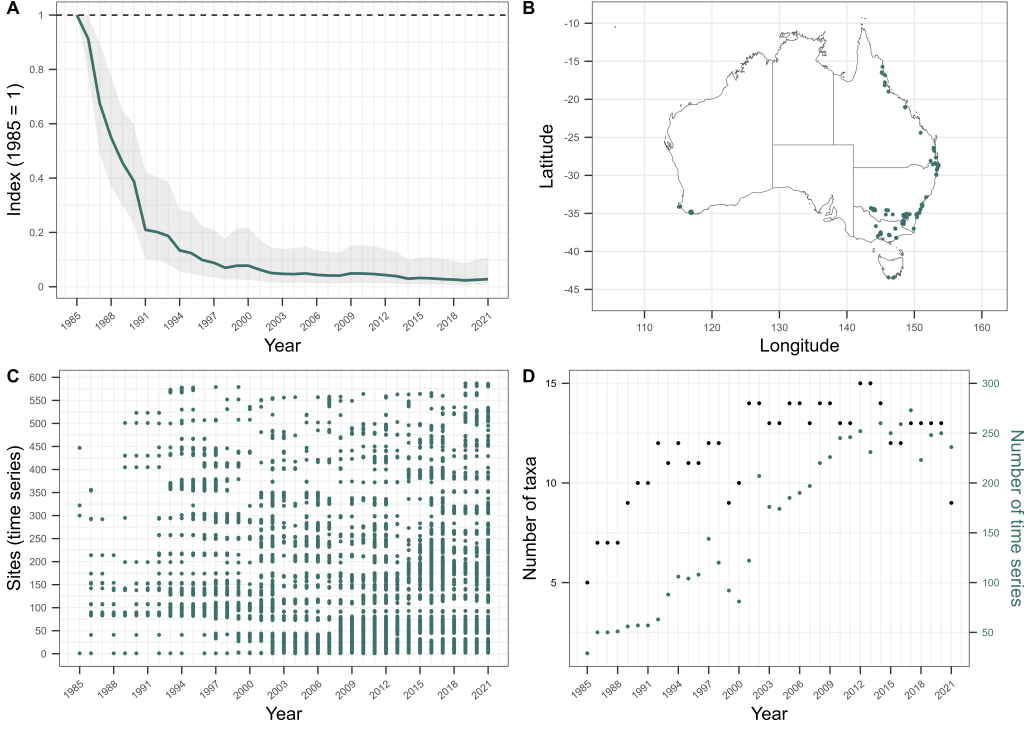
Sure enough, our matched bone samples revealed that the age structure of Bell Frogs is significantly truncated in remnant populations, with few frogs living for more than two years today, while historically a considerable proportion lived to four years of age or more. Using a fancy statistical approach developed by Mike Scroggie, we estimated that adult survival rates in remnant populations are 50% lower than prior to chytrid’s arrival.
Using this information as a basis, we then set about using metapopulation simulations to understand how this drop in survival rates constrains where these frogs can and cannot now live. We ran simulations of metapopulation trajectories varying fecundity rates (representing variation in habitat quality), annual variation in fecundity and survival rates (representing environmental stochasticity) and the number and proximity of habitat patches within a landscape (representing both habitat availability and connectivity). These simulations revealed that among the many combinations of these parameters, contemporary metapopulations of these frogs — with their depressed survival rates due to chytrid — could persist in much smaller zone of the parameter space than was historically the case. To survive, contemporary metapopulations needed high habitat quality (i.e., very high fecundity), low environmental stochasticity (low variation in fecundity and survival) and high habitat availability and connectivity. In short, our simulations revealed that chytrid has shrunk metapopulation viability for SBFs and GGBFs, to landscapes in which demographic resilience is at its highest — those with lots of top-quality habitat that provides fairly predictable conditions from one year to the next.
Of course, this all makes perfect sense and aligns with other studies on chytrid impacted frogs in Australia and elsewhere. But what this study contributes is a deeper understanding of the demographic mechanisms of persistence, and ultimately the environmental variables that underpin these mechanisms. In doing so, it gives us insights into the patterns of range contractions that Australian frogs have shown following the arrival of chytrid fungus to our shores, and it can be used to guide management to secure remnant populations. As we explain in the paper, if we can implement management that increases habitat availability and connectivity, increases recruitment rates and moderates the impact of environmental variation (like rainfall and temperature variability delivered by cycles of El Niño and La Niña), then we stand a good chance of enabling these populations to withstand chytrid-related mortality. Think of it this way — we can make these frogs resistant to the fungus, but through demographic levers, not immunological or evolutionary ones.
Ultimately, the hope is that the coming generations will be able to experience the extraordinary phenomena that Murray Littlejohn, Bill Sherwin and Bob Humphries experienced in the 1970s. I revel in the thought of future herpetologists wading through the wetlands of south-eastern Australia amid a cacophony of Bell Frogs. If we support them to out-breed chytrid and overcome the demographic hit from other threats — though habitat protection, enhancement and creation — we can make this a reality.






 Cold, wet, exhausted. It’s 2AM and you’re 1000 m up a rainforest ravine in the Wet Tropics. You’re finally horizontal, but your hammock – saturated and starting to smell of mildew – offers little prospect of sleep. Outside, in the pitch black, the stream you’ve been relentlessly climbing cascades over boulders that it has been wearing down for millennia. But over the white noise of water on granite, you can hear a frog calling “wreek wreek wreek”. It’s the Common Mistfrog, and you’ve just detected the species high atop the jungle-clad Mt Lewis. In the process, you’ve confirmed that this species is winning the battle against
Cold, wet, exhausted. It’s 2AM and you’re 1000 m up a rainforest ravine in the Wet Tropics. You’re finally horizontal, but your hammock – saturated and starting to smell of mildew – offers little prospect of sleep. Outside, in the pitch black, the stream you’ve been relentlessly climbing cascades over boulders that it has been wearing down for millennia. But over the white noise of water on granite, you can hear a frog calling “wreek wreek wreek”. It’s the Common Mistfrog, and you’ve just detected the species high atop the jungle-clad Mt Lewis. In the process, you’ve confirmed that this species is winning the battle against 

 Rainforest glamping? Not at 1000 m up a ravine, with blood oozing from myriad leech bites, battered shins and little more than gluggy porridge awaiting for breakfast.
Rainforest glamping? Not at 1000 m up a ravine, with blood oozing from myriad leech bites, battered shins and little more than gluggy porridge awaiting for breakfast. I suspect I’m one of many for which the predictability of species distributions was a gateway drug to ecology. My journey began with an extraordinary biogeographic transition point, delimited by the sheer slopes of the
I suspect I’m one of many for which the predictability of species distributions was a gateway drug to ecology. My journey began with an extraordinary biogeographic transition point, delimited by the sheer slopes of the 
 Road trips of my youth rarely entailed music. My siblings and I cruised the highways of south-eastern Australia mostly in silence. Melbourne to Bright – silence. Melbourne to Barmah – silence. Melbourne to Canberra – silence. Melbourne to Mildura – silence.
Road trips of my youth rarely entailed music. My siblings and I cruised the highways of south-eastern Australia mostly in silence. Melbourne to Bright – silence. Melbourne to Barmah – silence. Melbourne to Canberra – silence. Melbourne to Mildura – silence. Last week myself and colleagues from Charles Sturt University, Deakin University and the University of Queensland wrote a piece for
Last week myself and colleagues from Charles Sturt University, Deakin University and the University of Queensland wrote a piece for  How does one control a rapacious pathogen? If it were an infectious agent of humans, we would have much in our armoury. We could
How does one control a rapacious pathogen? If it were an infectious agent of humans, we would have much in our armoury. We could 
